Irrigation water is a key factor in the production of nursery and greenhouse crops. Therefore it is important to monitor quality standards on a frequent basis to avoid potential problems.
Often growers are unfamiliar with the many determinations that are made on a routine water test. This also makes interpretation of the results somewhat difficult. The following is a brief summary of these quality factors, as well as guidelines which may be used to determine their effect on plant growth.
Electrical Conductivity (EC) is a measure of the total salt content of water based on the flow of electrical current through the sample. The higher the salt content, the greater the flow of electrical current. EC is measured in mho/cm, which is the opposite of ohms of electrical resistance. Since the conductivity of most water is very low, EC is generally reported in thousandths of a mho or millimhos/cc.
Carbonate + Bicarbonate (CO3+ HCO3) are actually salts of carbonic acid (the acid formed when carbon dioxide dissolves in water). When in combination with calcium and/or magnesium (CaCO3, MgCO3 ) there is an alkalizing effect. This is generally mild because they are slightly soluble salts of moderately strong bases and weak acids. A stronger alkalizing effect may occur in the presence of sodium (Na2CO3) because this is a highly soluble salt of a strong base and weak acid. Carbonates and bicarbonates are reported in milliequivalents/ liter.
Calcium and Magnesium (Ca, Mg) are cations (positively charged ions) which are present in water. In most cases the sum of Ca and Mg are reported in milliequivalents/liter. Together Ca + Mg may be used to establish the relationship to total salinity and to estimate the sodium hazard.
Sodium (Na) is another cation occurring in most irrigation water. Along with Ca and Mg, Na is present in total amounts usually exceeding 0.1%. Sodium is often responsible for salinity problems when linked to chloride (Cl) and sulfate (SO4) but seldom from Ca or Mg. Sodium is expressed in terms of the sodium absorption ratio (SAR) calculated as follows: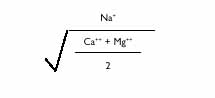
Chloride (Cl) is an anion (negatively charged ion) frequently occurring in irrigation water. Cl determinations are used to establish the relationship to total acidity as well as to indicate possible toxicities to sensitive crops.
Acidity/Alkalinity (pH) acids when mixed with water ionize into hydrogen ions (H+) and associated anions. The stronger the acid the greater the amount of ionization. Weak acids (such as those in irrigation water) generally ionize to less than 1.0%. The H+ ion activity of these acids is stated in terms of the logarithm of the reciprocal of H+ ion activity or pH.
Interpreting Water Quality
The quality of irrigation water is dependent on total salt content, the nature of salts present in solution and the proportion of Na to Ca, Mg, bicarbonates and other cations. The following table presents guidelines on the interpretation of the water quality factors.
| Quality | Electrical conductivity EC X 10 -3 (millimhos) | Total soluble Salts (ppm) | Sodium content (% Salts as Na) | SAR | pH |
|---|---|---|---|---|---|
| Excellent | 0.25 | 175 | 20 | 3 | 6.5 |
| Good | 0.25 – 0.75 | 175 – 525 | 20 – 40 | 3 – 5 | 6.5 – 6.8 |
| Permissible | 0.75 – 2.0 | 525 – 1400 | 40 – 60 | 5 – 10 | 6.8 – 7.0 |
| Doubtful | 2.0 – 3.0 | 1400 – 2100 | 60 – 80 | 10 – 15 | 7.0 – 8.0 |
| Unsuitable | >3.0 | >2100 | >80 | >15 | >8.0 |
For approximate conversion of EC to parts per million use the following calculations:
Millimhos
ppm = (EC x 10-3 ) x 670
Micromhos
ppm = (EC x 10-6 ) x 0.67
